Traceability has become a crucial element in production processes, particularly in the food and beverage industry. In sterilization processes using autoclaves, where ensuring the safety and compliance of products is essential, tracking each stage of the loading and unloading cycle is vital.
Thanks to the adoption of advanced digital technologies and the growing connectivity between machines and systems, it is now possible to monitor production parameters in real time, ensuring not only product quality but also regulatory compliance.
1. Product Traceability in Autoclave Loading and Unloading Processes
In food sterilization processes, traceability begins when products are loaded into the autoclave and continues through unloading. Each phase must be documented and monitored to ensure that the product meets safety and quality standards.
Key features:
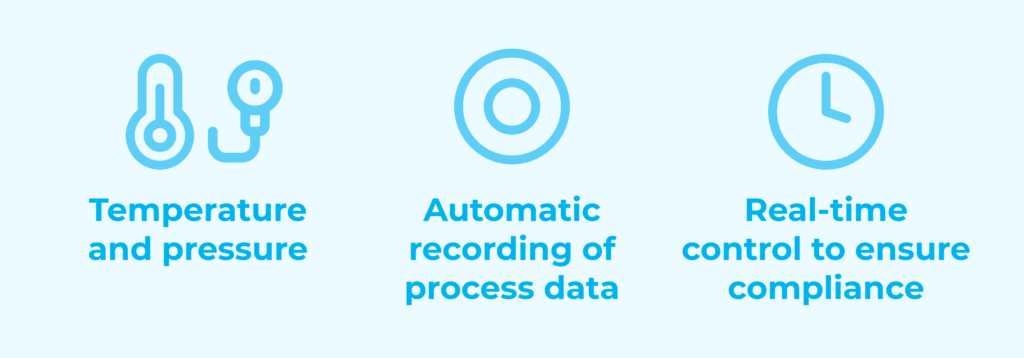
- Monitoring of temperature and pressure parameters.
- Automatic recording of process data.
- Real-time control to ensure compliance.
Benefits of traceability in autoclave loading/unloading:
- Improved product quality.
- Enhanced food safety.
- Greater transparency and access to production data.
Read also:Food product traceability in the supply chain is fundamental to ensure food safety
2. Quality Timer: Process Definition and Compliance
The quality timer is an essential tool for controlling the exposure times of products to specific sterilization parameters, such as temperature and pressure. It ensures that each batch receives the appropriate treatment without compromising product quality.
Key aspects of the quality timer:
- Precise measurement of sterilization times.
- Customization of the sterilization cycle based on product characteristics.
- Real-time monitoring to prevent errors.
Production advantages:
- Better control over quality parameters.
- Compliance with food safety standards.
- Reduction of production waste.
3. Batch Production Analysis and Management in Autoclave Loading/Unloading
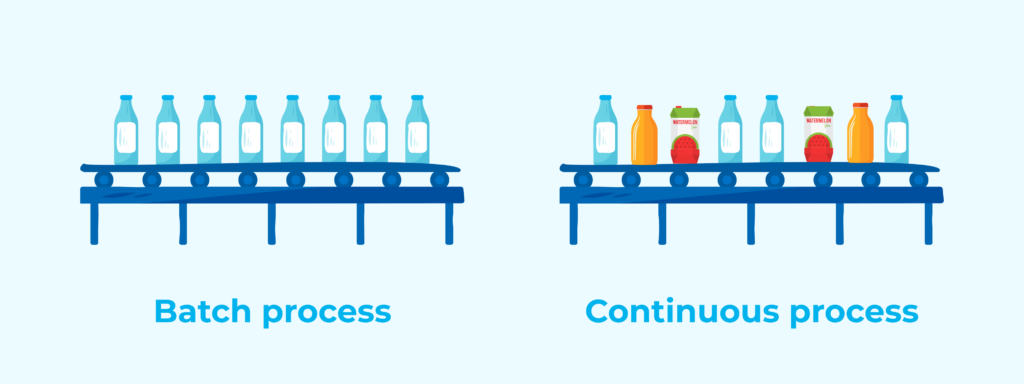
In the food industry, production can be managed through batch processes or continuous processes. In autoclave loading and unloading, batch traceability allows for precise control over each stage of the process.
Batch vs. continuous process:
- Batch process: Each group of products is treated separately and tracked individually.
- Continuous process: Products flow constantly through the system, requiring more advanced traceability.
Quality management in batch processes:
- Recording data for each sterilization cycle.
- Traceability of quality parameters for each batch.
- Real-time monitoring of operating conditions.
4. CFR 21 Certification According to FDA US Regulations
The CFR 21 Part 11 regulation established by the FDA (Food and Drug Administration) in the United States sets out requirements for managing electronic data and signatures, particularly relevant in the food industry to ensure product safety.
Compliance requirements:
- Electronic data management systems.
- Data security and integrity.
- Controlled access to data and complete traceability.
Connection with the PLC: The connection between the client server and the PLC (Programmable Logic Controller) via a unique network enables secure monitoring and management of each sterilization process stage, ensuring compliance with FDA requirements.
The advantages of a compliant system:
- Guaranteed food safety.
- Secure access to data.
- Traceability and regulatory compliance.
Conclusion
Traceability in autoclave loading and unloading processes is essential to ensure food safety and regulatory compliance. By using advanced technologies like the quality timer, batch traceability, and CFR 21 certification, companies can optimize their production processes, reduce risks, and ensure high-quality products.Want to improve traceability in your company? Contact us for a personalized consultation.